When diving into the world of CBD, purity and safety are paramount. But how can you be certain that your product meets the highest standards of quality? This is where a Certificate of Analysis (COA) becomes an indispensable tool. As a seal of approval, the CBD COA assures consumers of the product’s integrity, providing an in-depth snapshot of its composition. Understanding the CBD COA process is critical not only for compliance but also for maintaining unparalleled consumer confidence. With our comprehensive CBD COA guide, we’ll take you through the essentials of obtaining a COA and how it can add unmatched value to your CBD experience.
Key Takeaways
- Understand the critical role of a COA in validating the purity and quality of CBD products.
- A COA should contain essential data like active ingredients and batch-specific test results.
- Discover how manufacturers issue COAs in line with authoritative guidelines like those from the FDA.
- Realize the COA’s importance in fostering transparency and trust in CBD transactions.
- Learn about the detailed testing and information a COA offers compared to other certifications, such as the Certificate of Conformance (CoC).
- Explore the best practices for the CBD COA process, ensuring accurate and timely documentation.
 Understanding the Importance of CBD Certificate of Analysis (COA)
Understanding the Importance of CBD Certificate of Analysis (COA)
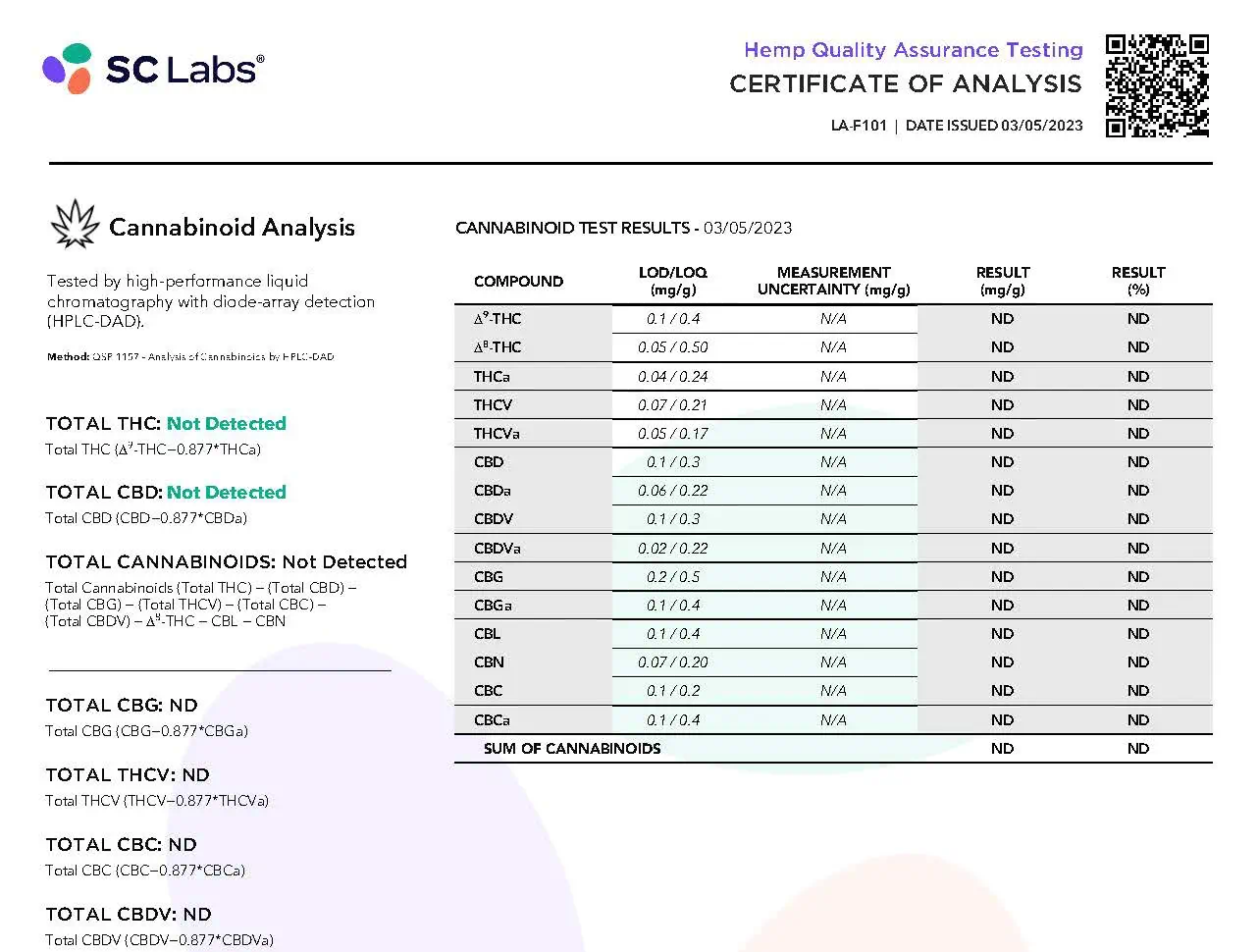
The distinction and importance of a Certificate of Analysis in the CBD industry cannot be understated. Often referred to as a COA, this document acts as a backbone for quality assurance and consumer safety. It is a pivotal factor influencing purchasing decisions and regulatory compliance, particularly in an industry where purity and potency are critical concerns. In this section, we will delve into the intricacies of COAs, their benefits, and how they differ from documents like the Certificate of Conformance.
What Is a Certificate of Analysis and Its Purpose?
A Certificate of Analysis is a document that verifies a CBD product meets specified criteria of quality. It is an essential tool that reflects the importance of CBD COA by providing transparency and confirming that the product has undergone rigorous testing to meet industry standards. The CBD certificate of analysis steps include detailed records of analysis conducted, ensuring everything from cannabinoid content to the absence of harmful contaminants is accounted for. Consumers rely on the accuracy of a COA to ensure the safety and efficacy of their CBD products.
The Benefits of Acquiring a COA for CBD Products
Having a COA is not just a mere formality; it offers numerous benefits to consumers and manufacturers alike. It gives customers peace of mind, knowing that what’s on the label matches what’s in the bottle. This vital document boosts consumer trust, potentially increasing brand loyalty and repeat purchases. From a business standpoint, presenting a COA can differentiate a product in a crowded market, suggesting a commitment to quality and compliance with CBD COA requirements.
Differentiating COA from Certificate of Conformance (CoC)
While a COA and a Certificate of Conformance (CoC) may serve similar purposes in validating product specifications, they are not interchangeable. A COA offers a deeper level of verification, typically involving specific test conditions, methodologies, and detailed results. It’s often produced by the manufacturer’s quality control department, providing a layer of accountability and traceability. In contrast, a CoC is more of a declaration, often sourced from external bodies and is less detailed. Below is a table illustrating key differences:
| Aspect | Certificate of Analysis (COA) | Certificate of Conformance (CoC) |
|---|---|---|
| Detail Level | High – Includes specific results | General – Confirms adherence to standards |
| Purpose | Verification of each batch’s specifications | Declaration that products meet basic requirements |
| Issuer | Typically in-house quality control | External laboratories or certification bodies |
| Used For | Ensuring quality, safety, and traceability | Broad declaration of conformity |
| Regulatory Use | Often required by agencies like the FDA | May not be accepted for all regulatory purposes |
Understanding these documents’ nuances empowers stakeholders to make informed decisions and fosters an environment of transparency and trust within the CBD marketplace.
 CBD How To Certificate Of Analysis COA: Navigating the COA Process
CBD How To Certificate Of Analysis COA: Navigating the COA Process
The procurement of a Certificate of Analysis (COA) is vital for anyone involved in the production or distribution of CBD products. A comprehensive CBD COA explained can reassure consumers about the product’s safety and quality, making it an indispensable tool in the CBD industry. To ensure the accuracy and efficiency of the COA process, there are several best practices that should be adhered to.
The initial step is the meticulous collection of supplier information, encompassing every detail from the origin of the materials to the adherence to industry standards. Once materials are identified, the transportation details logged, and product conformance verified, a signature from an authorized individual is required to validate the document. It is crucial for both the Quality Assurance (QA) and Quality Control (QC) teams to collaborate in harmonizing the steps involved in generating a COA to prevent any potential shipping delays.
One of the CBD COA best practices includes the adoption of Quality Management System (QMS) software. This innovation plays a pivotal role in enhancing the overall COA process by:
- Facilitating efficient data collection and management
- Standardizing the process of COA generation
- Maintaining secure digital archives of all COAs issued
By optimizing these processes with the help of QMS software, companies can not only ensure regulatory compliance but also improve the consistency and reliability of their COA documentation.
| COA Component | Description | Best Practices |
|---|---|---|
| Supplier Information | Details on the source and quality of materials | Thoroughly vet suppliers; maintain detailed records |
| Material Identification | Identification and verification of CBD raw materials | Implement batch testing and traceability protocols |
| Transport Details | Logistics information regarding the shipping of materials | Ensure secure and compliant transportation |
| Conformance Evidence | Proof of product meeting predefined specifications | Adopt rigorous testing methodologies |
| Signature Data | Authorization by a designated and competent individual | Use electronic signature capabilities for verification |
Adhering to these best practices not only assures compliance with stringent regulations but also establishes a robust foundation for the company’s reputation for quality assurance. Through a detailed CBD COA explained, stakeholders can navigate the complex landscape of CBD production with confidence, knowing that every product meets the high standards expected in the industry.
Conclusion
In concluding our exploration of the essentials of acquiring a Certificate of Analysis for CBD products, it’s clear that precision and stringent adherence to best practices are non-negotiable. As the industry continues to evolve, the emphasis on ensuring accurate COAs cannot be overstated. These documents not only validate product integrity and safety, but they also establish a foundation of trust between consumers, manufacturers, and regulatory entities such as the FDA, ISO, and OSHA.
Best Practices for Ensuring Accurate COAs
Best practices in the realm of COAs revolve around rigorous testing procedures, timely and meticulous record-keeping, and the embrace of technological solutions that minimize human error. Companies are encouraged to invest in accurate laboratory testing alongside maintaining comprehensive and up-to-date records that can stand up to the scrutiny of regulatory compliance. Successful implementation of these measures is essential for substantiating the claims made about any CBD product, ensuring that customers remain well-informed and confident in their purchases.
Adopting Streamlined Solutions for COA Management
The advancement of automated systems, illustrated by platforms such as Datacor ERP, demonstrates how efficiency can be significantly improved in generating and managing COAs. These systems handle the customization of COA documents to match the specifications of each customer and integrate all necessary tests to ensure they conform to regulatory compliance standards. Streamlined solutions enable companies to deliver COAs electronically, reducing the turnaround time and fostering higher levels of satisfaction among all stakeholders. Adopting such technologies not only helps in maintaining the integrity of the CBD COA process but also propels the industry toward a future where credibility and transparency are a given.

 CBD How To Certificate Of Analysis COA: Navigating the COA Process
CBD How To Certificate Of Analysis COA: Navigating the COA Process